|
|
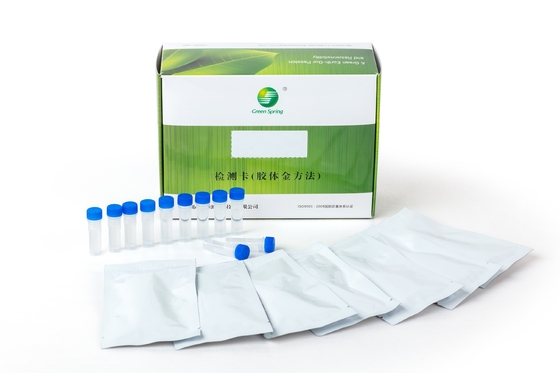
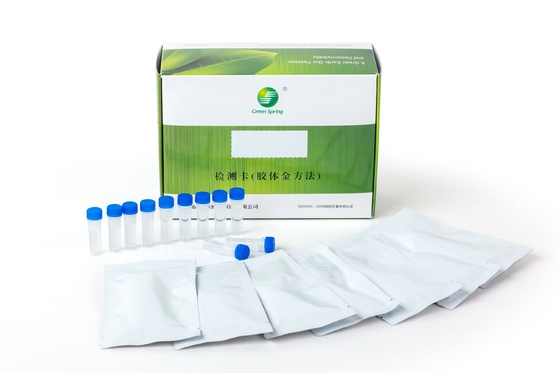
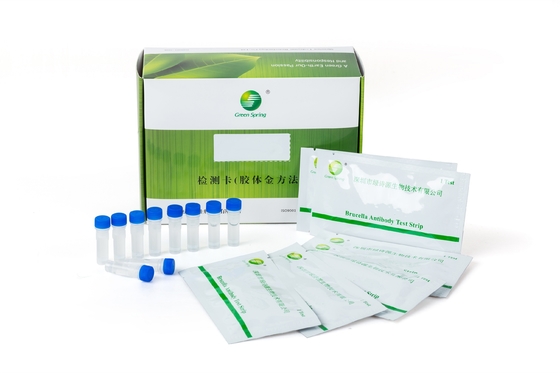
Group A streptococcus (Strep A) Test Kit (Colloidal Gold Method)
Product Details:
Payment & Shipping Terms:
|
| Packing: | 25 Servings/box | Specimens: | Throat Swab Specimens |
|---|---|---|---|
| Sensitivity: | 96.55% | Specificity: | 98.08% |
Group A streptococcus (Strep A) Test Kit (Colloidal Gold Method)
【Product Name】 Group A streptococcus (Strep A) Test Kit (Colloidal Gold Method)
【Packing Specifications】 25 servings/box
【Intended Use】
Strep A Test Kit is a rapid and convenient immunochromatographic assay for the qualitative detection of Group A streptococcus (Strep A) antigen from patient throat swab specimens. It is intended for professional use as an aid in the diagnosis of Strep A infection. This assay provides only a preliminary result. Clinical expertise and professional judgment should be sought to further evaluate the result of the test.
【Test Principle】
Streptococcus pyogenes is non-motile gram-positive cocci and known as Group A Streptococcus. The bacteria cause serious acute upper respiratory tract infections such as pharyngitis, respiratory infection, impetigo, endocarditis, meningitis, sepsis and arthritis. Identification procedures in laboratory for Strep A infection involve isolation of viable organisms, followed by various biochemical, microbiological (cell culture) or molecular biology techniques that usually require 24 to 48 hours or even longer. Early diagnosis and treatment of Group A Streptococcal pharyngitis has been shown to reduce the severity of symptoms and further complications such as rheumatic fever and glomerulonephritis. Strep A Test detects either viable or nonviable organisms directly from a throat swab, providing results within 10 minutes.
The test is an antigen-capture immunochromatographic assay, which detects the presence of Strep A in throat swab samples. Monoclonal antibodies specifically against Strep A are 1) conjugated with colloidal gold and deposited on the conjugate pad and 2) immobilized on the test line of the nitrocellulose membrane. When adequate volumes of the test sample is added the antibody conjugate is rehydrated and the Strep A, if any in the samples, will interact with the colloidal gold conjugated antibodies. The antigen-antibody-gold complex will migrate towards the test window until the Test Zone (T) where they will be captured by immobilized antibodies, forming a visible pink line (Test band), indicating a positive result. If Strep A are absent in the sample, no pink line will appear in the Test Zone (T), indicating a negative result.
To serve as an internal process control, a control line should always appear at Control Zone (C) after the test is completed. Absence of a pink control line in the Control Zone is an indication of an invalid result. The detection limit of Strep A Test Kit is approximately 105 Group A Streptococci organism.
【Main components】
- Pouch contents: Test cassette, desiccant, sample dropper.
- Sample extraction buffer A (7 ml per bottle for 25 tests), buffer B (8 ml per bottle for 25 tests).
- Test instruction.
【Storage and stability】
- Test device in the sealed pouch should be stored at 2~30°C. Do not freeze the test device.
- The bottle containing the buffer should be stored at 2~30°C.
- The test device should be kept away from direct sunlight, moisture and heat.
【Sample requirements】
- Swab the posterior pharynx, tonsils and other inflamed areas (Note: avoid touching the tongue, cheeks and teeth with the swab).
- A specimen should be collected by standard throat swab collection methods. It is preferable to use dacrontipped sterile swab with plastic shafts. Swabs should be processed as soon as possible after collection. If swabs are not processed immediately, they should be placed into a sterile, dry, tightly capped tube or bottle. Swabs which have been transported in liquid media such as modified Stuart's Transport Medium may be used in the test provided the liquid medium has a volume of 1 ml or less. Do not use semisolid transport media containing charcoal. Specimen swabs may be refrigerated (2-8 °C) for up to 5 days. If a culture is desired, lightly roll the swab tip onto a Group A selective blood agar plate before using the swab in the Strep A Rapid Test Device
- Add three full drops (85 µl) of specimen without air bubbles into the sample well that is marked with an arrow on the testing device.
[Basic Information]
Manufacturer: Shenzhen Lvshiyuan Biotechnology Co., Ltd
101,201,301, D Building, No.2 Industrial Avenue, Buxin Village, Buxin Community, Dapeng Subdistrict Office, Dapeng New District, Shenzhen, 518120 China
E-Mail: info@lsybt.com
EU Authorized Representative: Sungo Cert GmbH
Harffstr.47,40591 Dusseldorf, Germany
Tel:+49-211-97634133
E-mail:sungo.group@yahoo.com
-
Dengue NS1 Rapid Test Kit (Colloidal Gold) in vitro qualitative test of Dengue NS1 antigen in the sample of human serum.
-
Treponema Pallidum (TP) IgM/IgG Antibody Rapid Test Kit (Colloidal Gold) in vitro qualitative test of Treponema Pallidum
-
Dengue IgG/IgM Rapid Test Kit (Colloidal Gold) in vitro qualitative test of Dengue IgG/IgM antibody in the sample of hu
-
Luteinizing hormone (LH) Assay Kit (Fluorescence Immunochromatographic Assay)
-
Follicle Stimulating Hormone (FSH) Assay Kit (Fluorescence Immunochromatographic Assay)
-
Beta subunit of human chorionic gonadotropin (β-hCG) Assay Kit (Fluorescence Immunochromatographic Assay)